Alzheimer’s & Down syndrome research by Huntington Potter, PhD
 Huntington Potter, Ph.D., is Director of Alzheimer’s Disease Programs at the Linda Crnic Institute for Down Syndrome, Director of Rocky Mountain Alzheimer’s Disease Center, and the Professor and Vice Chair of Basic Research at the University of Colorado School of Medicine’s Department of Neurology. Potter discovered the mechanistic relationship between Alzheimer’s disease and Down syndrome. Below are selected excerpts from his groundbreaking research:
Huntington Potter, Ph.D., is Director of Alzheimer’s Disease Programs at the Linda Crnic Institute for Down Syndrome, Director of Rocky Mountain Alzheimer’s Disease Center, and the Professor and Vice Chair of Basic Research at the University of Colorado School of Medicine’s Department of Neurology. Potter discovered the mechanistic relationship between Alzheimer’s disease and Down syndrome. Below are selected excerpts from his groundbreaking research:
Recent Research
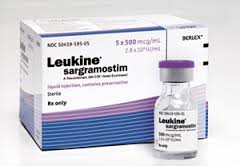 Dr. Potter’s recent research includes the GM-CSF treatment for Alzheimer’s, which involves the drug Luekine. GM-CSF is a protein that is secreted by patients with rheumatoid arthritis that may help explain why those patients rarely develop Alzheimer’s disease. Mice with Alzheimer’s disease that have been injected with Leukine were cured, and the invention is being proposed as a treatment for Alzheimer’s in humans, with clinical trials underway in Tampa and soon to follow in Denver.
Dr. Potter’s recent research includes the GM-CSF treatment for Alzheimer’s, which involves the drug Luekine. GM-CSF is a protein that is secreted by patients with rheumatoid arthritis that may help explain why those patients rarely develop Alzheimer’s disease. Mice with Alzheimer’s disease that have been injected with Leukine were cured, and the invention is being proposed as a treatment for Alzheimer’s in humans, with clinical trials underway in Tampa and soon to follow in Denver.
Leukine is already approved in humans as a bone-marrow stimulant. Potter’s invention would extend its use as a treatment for Alzheimer’s. Potter is the principal investigator on the grant for the Leukine experiments, along with the Linda Crnic Institute and the University of Colorado Denver.
American Journal of Human Genetics, June 1991
Review and hypothesis: Alzheimer disease and Down syndrome–chromosome 21 nondisjunction may underlie both disorders
Potter H.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1683102/
 In this publication, Dr. Potter became the first to hypothesize that there is a pathological relationship between Down syndrome, trisomy 21, and Alzheimer’s disease. Dr. Potter’s hypothesis suggested that both genetic and sporadic forms of Alzheimer’s disease can be explained as arising from the presence of small numbers of full trisomy 21 cells in apparently typical individuals. The accumulation of trisomy 21 cells in typical individuals, leading to the onset of Alzheimer’s disease, was proposed to be caused by defects in mitosis (i.e. the process that cells divide within the body), and is analogous to the defects in meiosis that cause individuals with Down syndrome to be born with trisomy 21 (i.e. having three copies of chromosome 21 within their cells), and who are known to develop pathology in their brain that is indistinguishable from the pathology found in the brains of Alzheimer’s disease patients.
In this publication, Dr. Potter became the first to hypothesize that there is a pathological relationship between Down syndrome, trisomy 21, and Alzheimer’s disease. Dr. Potter’s hypothesis suggested that both genetic and sporadic forms of Alzheimer’s disease can be explained as arising from the presence of small numbers of full trisomy 21 cells in apparently typical individuals. The accumulation of trisomy 21 cells in typical individuals, leading to the onset of Alzheimer’s disease, was proposed to be caused by defects in mitosis (i.e. the process that cells divide within the body), and is analogous to the defects in meiosis that cause individuals with Down syndrome to be born with trisomy 21 (i.e. having three copies of chromosome 21 within their cells), and who are known to develop pathology in their brain that is indistinguishable from the pathology found in the brains of Alzheimer’s disease patients.
Neurobiology of Disease, June 1999
Chromosome missegregation and trisomy 21 mosaicism in Alzheimer’s disease
Geller LN, Potter H.
http://www.ncbi.nlm.nih.gov/pubmed/10408806
In this publication, Dr. Potter addressed whether Alzheimer’s disease patients harbor an elevated frequency of aneuploid, specifically trisomy 21, cells. Aneuploidy is the process of the abnormal division of cells, in which duplicate chromosomes do not separate correctly, termed “missegregation,” and this leads to one resultant daughter cell having an extra copy of a chromosome, termed trisomy, and the other daughter cell having only one copy of the chromosome, termed monosomy. Monosomy cells do not survive, and the accumulation of a small number of trisomic cells within the body, harboring three copies of chromosome 21, is called “trisomy 21 mosaicism.” Dr. Potter reported within this publication that cells from both sporadic and familial Alzheimer’s disease patients will divide with a higher frequencies of trisomy 21 cells, as compared with individuals who do not have Alzheimer’s. Dr. Potter also reported that Presenilin proteins are involved in the process of cell division, and because it was known that mutations in the gene that encodes the Presenilin proteins cause early onset familial Alzheimer’s disease (FAD), suggested that FAD mutant Presenilin may also predispose to chromosome missegregation.
Neurobiology of Aging, May-June 2000
Cell cycle and chromosome segregation defects in Alzheimer’s disease
Potter H.
http://www.ncbi.nlm.nih.gov/books/NBK6373/
 In this publication, Dr. Potter summarized the relationships between Down syndrome and Alzheimer’s disease. Dr. Potter reported that individuals with Down syndrome and Alzheimer’s have similar hypersensitive alterations in their eyes, which was predicted by his 1991 hypothesis that individuals with Down syndrome and trisomy 21 mosaicism in Alzheimer’s patients would share other clinical features besides dementia. Furthermore, the most important prediction of the hypothesis, in which Alzheimer’s patients should accumulate trisomy 21 mosaicism, had been reported in Dr. Potter’s publication in 1999. These findings allowed Dr. Potter to make two further testable predictions, in which 1) alterations to the cellular machinery that allow a cell to divide should lead to chromosome missegregation and trisomy 21 mosaicism, and 2) mutations that cause Alzheimer’s should occur in genes that encode for proteins which are directly or indirectly involved in mitosis and chromosome segregation.
In this publication, Dr. Potter summarized the relationships between Down syndrome and Alzheimer’s disease. Dr. Potter reported that individuals with Down syndrome and Alzheimer’s have similar hypersensitive alterations in their eyes, which was predicted by his 1991 hypothesis that individuals with Down syndrome and trisomy 21 mosaicism in Alzheimer’s patients would share other clinical features besides dementia. Furthermore, the most important prediction of the hypothesis, in which Alzheimer’s patients should accumulate trisomy 21 mosaicism, had been reported in Dr. Potter’s publication in 1999. These findings allowed Dr. Potter to make two further testable predictions, in which 1) alterations to the cellular machinery that allow a cell to divide should lead to chromosome missegregation and trisomy 21 mosaicism, and 2) mutations that cause Alzheimer’s should occur in genes that encode for proteins which are directly or indirectly involved in mitosis and chromosome segregation.
Neurobiology of Aging, March 2008
Alzheimer’s Presenilin 1 causes chromosome missegregation and aneuploidy
Boeras DI, Granic A, Padmanabhan J, Crespo NC, Rojiani AM, Potter H.
http://www.ncbi.nlm.nih.gov/pubmed/17169464
In this publication, Dr. Potter follows up his work from the suggestions of his 1999 publication, and he examines whether FAD mutations in the Presenilin proteins indeed cause chromosomes to incorrectly segregate during cell division. Mutations in the Presenilin 1 and Presenilin 2 proteins cause the majority of FAD cases, with mutant Presenilin 1 contributing most. Normally the Presenilin proteins are part of the enzyme complex, called Gamma-Secretase, which in Alzheimer’s, helps to clip out the toxic Aβ peptide from the APP protein. When there are mutations in the Presenilin proteins, the Gamma-Secretase complex makes more of the toxic Aβ peptides, which then leads to early deposition of amyloid plaques in the brain and early onset of FAD, sometimes before the 4th or 5th decade of life in people who carry these mutations. Herein, Dr. Potter actually demonstrates that FAD-mutant Presenilin-1 proteins cause the increased frequency of aneuploidy in animal models of Alzheimer’s and within human cell cultures, including the generation of trisomy 21 cells.
Molecular Biology of the Cell, December 2010
Alzheimer Aβ Peptide Induces Chromosome Mis-Segregation and Aneuploidy, Including Trisomy 21: Requirement for Tau and APP
Granic A, Padmanabhan J, Norden M, Potter H.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2820417/
 In this publication, Dr. Potter reported that FAD mutations in the Amyloid Precursor Protein (APP) gene, which resides on chromosome 21 and is overproduced in people with Down syndrome, could cause chromosome missegregation and aneuploidy in transgenic animal models of Alzheimer’s and within cells containing these mutations. The pathogenic part of the APP protein, the Aβ peptide which is clipped out and causes the amyloid plaques in Alzheimer’s, was also shown to require the tau protein for the induction of aneuploidy. Inside of neurons, the tau protein is known to stabilize the microtubule tracks that are utilized for transport of molecules to and from the cell’s nucleus, and in Alzheimer’s, this tau protein comes off of the microtubules and clumps together into neurofibrillary tangles, which causes the neurons to be nonfunctional and then die.
In this publication, Dr. Potter reported that FAD mutations in the Amyloid Precursor Protein (APP) gene, which resides on chromosome 21 and is overproduced in people with Down syndrome, could cause chromosome missegregation and aneuploidy in transgenic animal models of Alzheimer’s and within cells containing these mutations. The pathogenic part of the APP protein, the Aβ peptide which is clipped out and causes the amyloid plaques in Alzheimer’s, was also shown to require the tau protein for the induction of aneuploidy. Inside of neurons, the tau protein is known to stabilize the microtubule tracks that are utilized for transport of molecules to and from the cell’s nucleus, and in Alzheimer’s, this tau protein comes off of the microtubules and clumps together into neurofibrillary tangles, which causes the neurons to be nonfunctional and then die.
Cell Cycle, May 2011
Alzheimer Aβ disrupts the mitotic spindle and directly inhibits mitotic microtubule motors
Borysov SI, Granic A, Padmanabhan J, Walczak CE, Potter H.
http://www.ncbi.nlm.nih.gov/pubmed/21566458
In this publication, Dr. Potter reported that the toxic Aβ peptide, which forms the amyloid plaques in Alzheimer’s, can cause the cellular machinery, which the cell uses to divide, to become nonfunctional and greatly altered. For a cell to divide properly and correctly separate chromosomes, it uses proteins to pull the duplicated chromosomes equally toward each new daughter cell. These proteins are called microtubule motors, and Dr. Potter found that the Aβ peptide inhibits some of these motors. Furthermore, these same motors also transport molecules from the nucleus of the cell towards the neuron’s dendritic projections. Therefore, Dr. Potter found that the FAD mutant APP, which makes the toxic Aβ peptide, can not only cause aneuploidy in dividing cells, but it can also severely inhibit the functioning of existing neurons within the brain.
Neurobiology of Aging, August 2014
Alzheimer amyloid beta inhibition of Eg5/kinesin 5 reduces neurotrophin and/or transmitter receptor function
Ari C, Borysov SI, Wu J, Padmanabhan J, Potter H.
http://www.ncbi.nlm.nih.gov/pubmed/24636920
In this publication, Dr. Potter demonstrated that when the toxic Aβ peptides inhibit the microtubule motor, called Eg5 or kinesin 5, this inhibition prevented certain critical proteins, which are made in the cell nucleus, from being transported to the outside of the neuron’s cell surface. These “neurotrophin” proteins and “transmitter receptors” are used by neurons to communicate with each other and to help protect them and other surrounding neurons from dying. This publication follows Dr. Potter’s earlier work, which showed that the toxic Aβ peptide will cause chromosome missegregation and trisomy 21 (Potter 2010 and 2011), and that it also can disrupt the normal functioning of neurons. Furthermore, Dr. Potter showed in this publication that when Aβ inhibited Eg5 and its transport of neurotrophic proteins and receptors to the cell surface, that this also inhibited long-term potentiation of the cell, which is a cellular model for learning and memory. Thus, Dr. Potter’s results in this publication established a very important understanding about the mechanisms that may underlie the onset of Alzheimer’s pathology and memory problems within individuals with Down syndrome.

 Experience our inspirational and groundbreaking videos and photos. Our children and self-advocates are beautiful AND brilliant!
Experience our inspirational and groundbreaking videos and photos. Our children and self-advocates are beautiful AND brilliant! Make sure your local Representatives are on the Congressional Down Syndrome Task Force.
Make sure your local Representatives are on the Congressional Down Syndrome Task Force.